About the Seminar:
The luminescence of lanthanide ions is based on f-f transitions. Due to the core nature of the 4f orbitals involved in the process, as well as the forbidden nature of these transitions, the emission properties make these ions uniquely suited for a variety of applications involving light emission, such as lighting, imaging, and sensing. Since the f-f transitions are forbidden, the emission is most efficiently promoted through coordinated chromophores. The use of these coordinated ligands provides unique opportunities. They can be functionalized to tailor the chemical and photophysical properties of the resulting complexes.1 We have used this approach to synthesize complexes that can be used as imaging agents for cancer cells.2 By extending the conjugation of the ligand we shifted the excitation wavelengths into the visible and isolated complexes that can be used as molecular nanothermometers.3 In addition, we used carbazole-based ligands that enabled excitation of the resulting complexes in the biological window by a two-photon process. Finally, we used oligothiophene-based ligands to isolate complexes that luminesce and generate singlet oxygen. These compounds have shown appreciable phototoxicity when incubated with HeLa cell. In this presentation, I will discuss my group’s recent work on lanthanide ion complexes with dual activity.
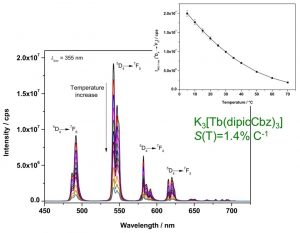
Figure 1. Temperature-dependent emission spectrum of K3[Tb(dipicCbz)3]. Inset shows the intensity of the 5D4 7F5 transition as a function of temperature.3
References
1. Luminescence of Lanthanide Ions in Coordination Compounds and Nanomaterials. de Bettencourt-Dias, A., Ed. John Wiley and Sons: 2014.
2. Monteiro, J. H. S. K.; Machado, D.; de Hollanda, L. M.; Lancellotti, M.; Sigoli, F. A.; de Bettencourt-Dias, A., Selective Cytotoxicity and Luminescence Imaging of Cancer Cells with A Dipicolinato-based EuIII Complex Chem. Commun. 2017, 53, 11818-11821.
3. Monteiro, J. H. S. K.; Sigoli, F. A.; de Bettencourt-Dias, A., A Water-soluble TbIII complex as temperature-sensitive luminescent probe. Can. J. Chem. 2018, 96, 859-864 (special issue of the Electron Donor-Accepter Interactions Gordon Research Conference).
About the Speaker:
Ana de Bettencourt-Dias received her ‘licenciatura’ (MS equivalent) in Technological Chemistry from the University of Lisbon in 1993, and her ‘Dr. rer. nat.’ (PhD equivalent) in Inorganic Chemistry from the University of Cologne in 1997 with Prof. Thomas Kruck. In her graduate work, she isolated new titanium complexes as single source precursors for the chemical vapor deposition of TiN thin layers. She joined the group of Alan Balch at UC Davis in 1998 as a Gulbenkian postdoctoral fellow, where she studied the electrochemistry and structure of fullerenes and endohedral fullerenes. In 2001 she joined the faculty at Syracuse University and started her work on luminescent lanthanide ion complexes. She moved to the University of Nevada, Reno, as associate professor in 2007 and was promoted to professor in 2013. At UNR she continues to pursue her research interests in the f block of the periodic table. She served as the Associate Vice President for Research at UNR from 2015 to 2019 and is the 2019 Chair of the Division of Inorganic Chemistry of the American Chemical Society. She returned to being a full-time faculty in July 2019, and is now the Susan Magee & Gary Clemons Professor of Chemistry.



